
سعر فوب
أحصل على آخر سعر|
1 Minimum Order
بلد:
Hong Kong
نموذج رقم:
-
سعر فوب:
الموقع:
-
سعر الحد الأدنى للطلب:
-
الحد الأدني للطلب:
1
تفاصيل التغليف:
-
موعد التسليم:
-
القدرة على التوريد:
-
نوع الدفع:
-
مجموعة المنتج :
-
الشخص الذي يمكن الاتصال به Angel
Hong Kong, Sheung Wan
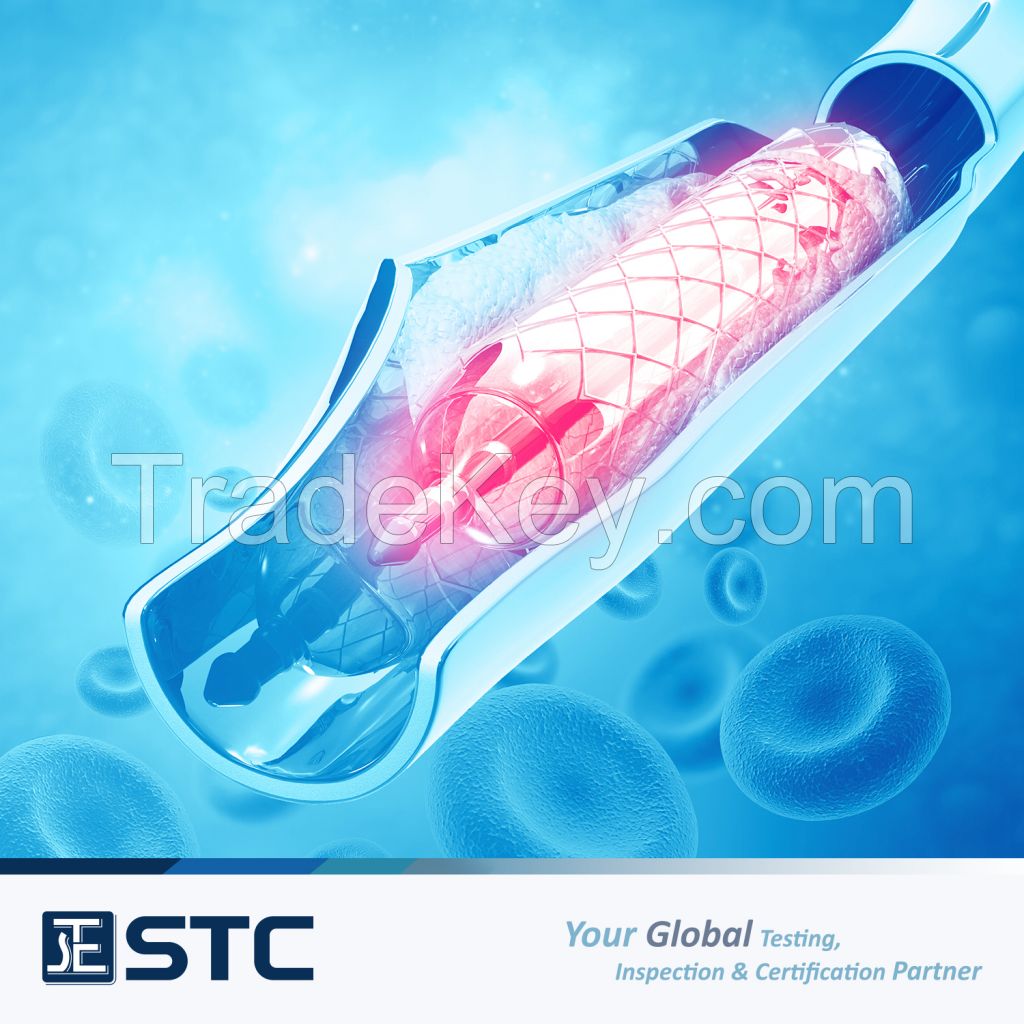
At STC, our team of knowledgeable experts are up-to-date with the
latest regulatory requirements in key markets to ensure that
compliance is met and support medical device manufacturers in
gaining market entry quickly.
Key Market Support:
Our Medical Device Product Classification & Regulatory Support services include:
Electrical Testing
Biological Testing (ISO ****3)
LUCIDEON Medical Device Services
If you have any further question, please feel free contact us.
| بلد: | Hong Kong |
| نموذج رقم: | - |
| سعر فوب: | أحصل على آخر سعر |
| الموقع: | - |
| سعر الحد الأدنى للطلب: | - |
| الحد الأدني للطلب: | 1 |
| تفاصيل التغليف: | - |
| موعد التسليم: | - |
| القدرة على التوريد: | - |
| نوع الدفع: | - |
| مجموعة المنتج : | - |