


سعر فوب
أحصل على آخر سعر( Negotiable )
|50000 Piece Minimum Order
بلد:
Others1
نموذج رقم:
11901
سعر فوب:
( Negotiable ) أحصل على آخر سعر
الموقع:
Others1
سعر الحد الأدنى للطلب:
-
الحد الأدني للطلب:
50000 Piece
تفاصيل التغليف:
as per attach photo or brochure
موعد التسليم:
As per cutomer requirements
القدرة على التوريد:
300000 Piece per Day
نوع الدفع:
T/T
مجموعة المنتج :
الشخص الذي يمكن الاتصال به Mr. Sam
Room 302C, No.718, Blv San Yuan Li, Bai Yuan District,, Changping, Guangdong
| بلد: | Others1 |
| نموذج رقم: | 11901 |
| سعر فوب: | ( Negotiable ) أحصل على آخر سعر |
| الموقع: | Others1 |
| سعر الحد الأدنى للطلب: | - |
| الحد الأدني للطلب: | 50000 Piece |
| تفاصيل التغليف: | as per attach photo or brochure |
| موعد التسليم: | As per cutomer requirements |
| القدرة على التوريد: | 300000 Piece per Day |
| نوع الدفع: | T/T |
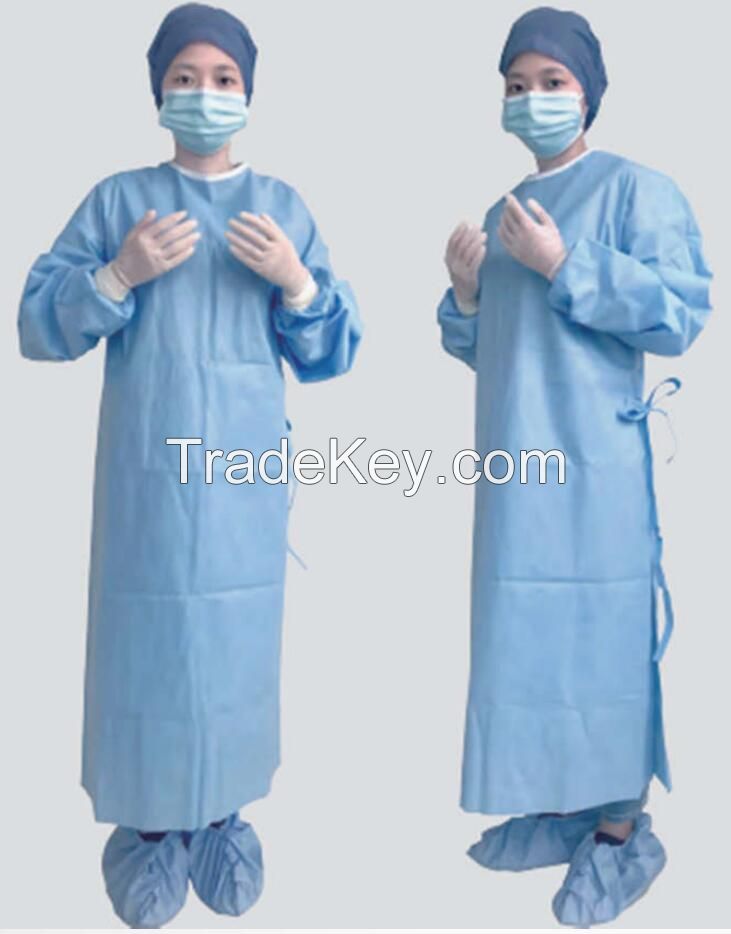
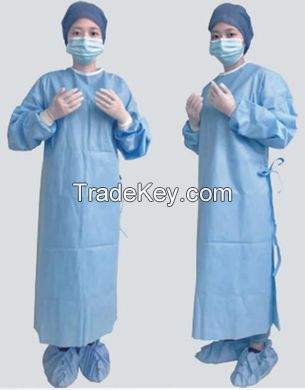
| مجموعة المنتج : | surgical suit |