FOB Price
أحصل على آخر سعر3.9 ~ 5.2 / Piece ( Negotiable )
|Minimum Order
Place of Origin:
-
Price for Minimum Order:
Minimum Order Quantity:
2000 Piece
Packaging Detail:
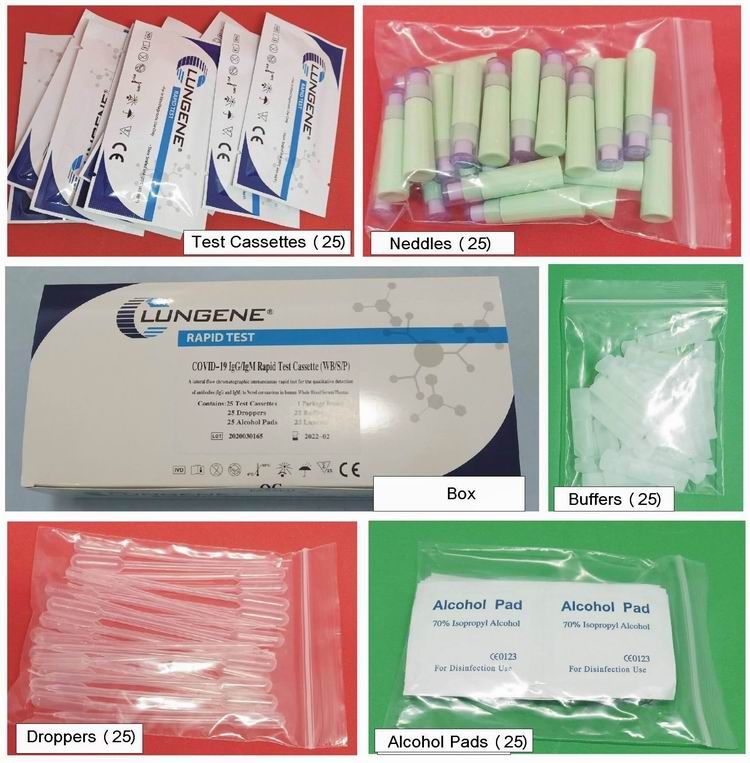
25pcs/box; 2000pcs/carton
Delivery Time:
5-7days after payment
Supplying Ability:
100000 Piece per Week
Payment Type:
T/T
الشخص الذي يمكن الاتصال به Kevin
Longlin, Shanghai, Shanghai
| COVID**9 IgM | RT-PCR | Total | ||
| Positive | Negative | |||
| CLUGENE | Positive | *7 | 1 | *8 |
| Negative | *0 | *9 | *9 | |
| Total | *7 | *0 | **7 | |
| COVID**9 IgG | Number of patients during the convalescence period | Total | |
| CLUGENE | Positive | *5 | *5 |
| Negative | 2 | 2 | |
| *7 | *7 | ||
|
Analytes
|
Conc.
|
Specimens
|
|
|
Positive
|
Negative
|
||
|
Albumin
|
*0mg/ml
|
+
|
-
|
|
Bilirubin
|
*0ug/ml
|
+
|
-
|
|
Hemoglobin
|
*5mg/ml
|
+
|
-
|
|
Glucose
|
*0mg/ml
|
+
|
-
|
| Uric Acid |
**0ug/ml
|
+
|
-
|
|
Lipids
|
*0mg/ml
|
+
|
-
|
| Analytes |
Conc (ug/ml) |
Specimens | |
| Positive | Negative | ||
| Acetaminophen | **0 | + | - |
| Acetoacetic Acid | **0 | + | - |
| Acetylsalicylic Acid | **0 | + | - |
| Benzoylecgonine | **0 | + | - |
| Caffeine | **0 | + | - |
| EDTA | **0 | + | - |
| Ethanol | 1.0% | + | - |
| Gentisic Acid | **0 | + | - |
| β-Hydroxybutyrate | ****0 | + | - |
| Methanol | *0.0% | + | - |
| Phenothiazine | **0 | + | - |
| Phenylpropanolamine | **0 | + | - |
| Salicylic Acid | **0 | + | - |